搜索结果: 'methocult media formulations for human hematopoietic cells serum containing'
-
 A Novel Animal Component-Free Culture System for Isolation and Expansion of Human Bone Marrow-Derived Mesenchymal Cells
A Novel Animal Component-Free Culture System for Isolation and Expansion of Human Bone Marrow-Derived Mesenchymal Cells产品类型:
Conference:
ISSCR 2013
产品号#:
产品名:
-
 A New Complete Animal Component-Free System for the Proliferation and Differentiation of Human Neural Stem and Progenitor Cells
A New Complete Animal Component-Free System for the Proliferation and Differentiation of Human Neural Stem and Progenitor Cells产品类型:
Conference:
SFN 2008
产品号#:
产品名:
-
 A Fully Defined Animal Component Free Medium for Efficient Differentiation of Human Pluripotent Stem Cells to Definitive Endoderm
A Fully Defined Animal Component Free Medium for Efficient Differentiation of Human Pluripotent Stem Cells to Definitive Endoderm产品类型:
Conference:
KEYSTONE 2012
产品号#:
产品名:
-
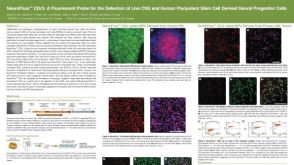 Neurofluor CDr3 a Fluorescent Probe for the Detection of Live CNS and Human Pluripotent Stem Cell Derived Neural Progenitor Cells
Neurofluor CDr3 a Fluorescent Probe for the Detection of Live CNS and Human Pluripotent Stem Cell Derived Neural Progenitor Cells产品类型:
Conference:
SFN 2014
产品号#:
05701
05702
05832
05833
05835
05839
08581
08582
产品名:
NeuroCult™ 扩增添加物 (小鼠&大鼠)
NeuroCult™ 扩增试剂盒 (小鼠&大鼠)
STEMdiff™ 神经花环选择试剂
STEMdiff™神经前体细胞培养基
STEMdiff™ 神经诱导培养基
STEMdiff™ 神经诱导培养基
STEMdiff™SMADi神经诱导试剂盒
STEMdiff™SMADi神经诱导试剂盒,2套
-
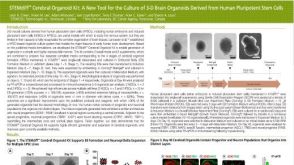 科学海报STEMdiff Cerebral Organoid Kit: A New Tool for the Culture of 3D Brain Organoids Derived from Human Pluripotent Stem Cells
科学海报STEMdiff Cerebral Organoid Kit: A New Tool for the Culture of 3D Brain Organoids Derived from Human Pluripotent Stem Cells产品类型:
Cell Culture Media and Supplements
Conference:
SFN 2017
产品号#:
08570
08571
产品名:
STEMdiff™ 脑类器官试剂盒
STEMdiff™ 脑类器官成熟试剂盒
-
 科学海报Stroma-Free, Serum-Free Expansion and Differentiation of Hematopoietic Stem and Progenitor Cells to the T Cell Lineage
科学海报Stroma-Free, Serum-Free Expansion and Differentiation of Hematopoietic Stem and Progenitor Cells to the T Cell Lineage产品类型:
Cell Culture Media and Supplements
Conference:
AAi 2017
产品号#:
09605
产品名:
StemSpan™ SFEM II
-
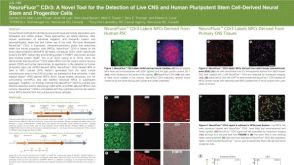 NeuroFluor™ CDr3: A Novel Tool for the Detection of Live CNS and Human Pluripotent Stem Cell-Derived Neural Stem and Progenitor Cells
NeuroFluor™ CDr3: A Novel Tool for the Detection of Live CNS and Human Pluripotent Stem Cell-Derived Neural Stem and Progenitor Cells产品类型:
Conference:
CAN 2015
产品号#:
01800
05701
05702
05832
05833
05835
05839
08581
08582
产品名:
NeuroCult™ 扩增添加物 (小鼠&大鼠)
NeuroCult™ 扩增试剂盒 (小鼠&大鼠)
STEMdiff™ 神经花环选择试剂
STEMdiff™神经前体细胞培养基
STEMdiff™ 神经诱导培养基
STEMdiff™ 神经诱导培养基
STEMdiff™SMADi神经诱导试剂盒
STEMdiff™SMADi神经诱导试剂盒,2套
-
 Human Hematopoietic Stem and Progenitor Cell Phenotyping
Human Hematopoietic Stem and Progenitor Cell Phenotyping产品类型:
产品号#:
产品名:
-
 1:58
NeuroCult™ Proliferation Media
1:58
NeuroCult™ Proliferation Media产品类型:
产品号#:
05700
05702
05751
05761
产品名:
NeuroCult™ 基础培养基(小鼠&大鼠)
NeuroCult™ 扩增试剂盒 (小鼠&大鼠)
NeuroCult™ NS-A 扩增试剂盒(人)
用于小鼠和大鼠神经干细胞和祖细胞分化培养的试剂盒
发布日期: 1/19/11 -
 科学海报A Serum-Free Workflow for the Isolation, Expansion and Differentiation of Human Myogenic Progenitors
科学海报A Serum-Free Workflow for the Isolation, Expansion and Differentiation of Human Myogenic Progenitors产品类型:
Conference:
Myogenesis GRC 2017,Till and McCulloch Meeting 2017
产品号#:
05980
05982
05983
产品名:
MyoCult™-SF 扩增添加物试剂盒 (人)
MyoCult™-SF 扩增10X添加物(人)
MyoCult™-SF 贴附基质


 EasySep™小鼠TIL(CD45)正选试剂盒
EasySep™小鼠TIL(CD45)正选试剂盒







 沪公网安备31010102008431号
沪公网安备31010102008431号