搜索结果: 'methocult media formulations for human hematopoietic cells serum containing'
-
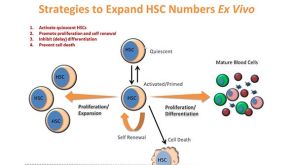 1:06:52
New Tools for the Ex Vivo Expansion of Human Hematopoietic Stem and Progenitor Cells
1:06:52
New Tools for the Ex Vivo Expansion of Human Hematopoietic Stem and Progenitor Cells产品类型:
产品号#:
02690
02691
02693
02696
02697
02692
09600
09650
09605
09655
04034
04044
72332
22001
22005
22006
22007
22008
22009
22011
22012
78129.1
78129.2
78135
78135.1
78135.2
78137
78137.1
78137.2
78138
78138.1
78138.2
78140
产品名:
StemSpan™CC100
StemSpan™CD34+扩增补充(10X)
StemSpan™髓系扩增补品(100X)
StemSpan™巨核细胞扩增补充(100X)
StemSpan™CC110
StemSpan™红血系扩增补充(100X)
StemSpan™ SFEM
StemSpan™ SFEM
StemSpan™ SFEM II
StemSpan™ SFEM II
MethoCult™H4034 Optimum
MethoCult™H4034 Optimum
UM729
STEMvision™ 人脐带血7-天CFU分析包
STEMvision™ 彩色人脐带血14-天CFU分析包
STEMvision™ 彩色人骨髓14-天CFU分析包
STEMvision™ 彩色人动员外周血14-天CFU分析包
STEMvision™ 小鼠总CFU分析包
STEMvision™ 小鼠髓系CFU分析包
STEMvision™ 小鼠红系CFU分析包
STEMvision™ 小鼠CFU分析包(髓系和红系)
重组小鼠IL-1 α
重组小鼠IL-1 α
人/小鼠重组BMP-2
人/小鼠重组BMP-2
人/小鼠重组BMP-2
重组人Flt3/Flk-2 Ligand细胞因子, ACF
人重组Flt3/Flk-2配体
人重组Flt3/Flk-2配体
重组人G-CSF细胞因子, ACF
人重组G-CSF,ACF
人重组G-CSF,ACF
人重组GM-CSF,ACF
发布日期: 3/9/15 -
 协议Robust Scale-Up of Human Pluripotent Stem Cells Using TeSR™ 3D Media
协议Robust Scale-Up of Human Pluripotent Stem Cells Using TeSR™ 3D Media产品类型:
研究方向:
干细胞生物学,细胞治疗开发
产品号#:
产品名:
-
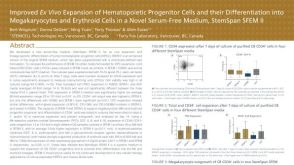 Improved Ex Vivo Expansion of Hematopoietic Progenitor Cells from Cord Blood in a Novel Serum and Animal Component Free Medium
Improved Ex Vivo Expansion of Hematopoietic Progenitor Cells from Cord Blood in a Novel Serum and Animal Component Free Medium产品类型:
Conference:
ISCT 2012
产品号#:
产品名:
-
 科学海报Stroma-Free, Serum-Free Expansion and Differentiation of Hematopoietic Stem and Progenitor Cells to the T Cell Lineage
科学海报Stroma-Free, Serum-Free Expansion and Differentiation of Hematopoietic Stem and Progenitor Cells to the T Cell Lineage产品类型:
Cell Culture Media and Supplements
Conference:
AAi 2017
产品号#:
09605
产品名:
StemSpan™ SFEM II
-
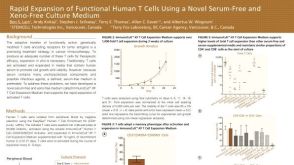 Rapid Expansion of Functional Human T Cells Using a Novel Serum-Free and Xeno-Free Culture Medium
Rapid Expansion of Functional Human T Cells Using a Novel Serum-Free and Xeno-Free Culture Medium产品类型:
Conference:
CCIC 2015
产品号#:
10970
10990
10971
10991
10981
产品名:
ImmunoCult™ 人CD3/CD28/CD2 T细胞激活剂
ImmunoCult™ 人CD3/CD28/CD2 T细胞激活剂
ImmunoCult™ 人CD3/CD28 T细胞激活剂
ImmunoCult™ 人CD3/CD28 T细胞激活剂
ImmunoCult™ XF 人T细胞扩增培养基,500 mL
-
 Workflow Solutions for Human T Cell Isolation and Expansion
Workflow Solutions for Human T Cell Isolation and Expansion产品类型:
Conference:
CCIC 2015
产品号#:
10970
10990
10971
10991
10981
17951
17951RF
产品名:
ImmunoCult™ 人CD3/CD28/CD2 T细胞激活剂
ImmunoCult™ 人CD3/CD28/CD2 T细胞激活剂
ImmunoCult™ 人CD3/CD28 T细胞激活剂
ImmunoCult™ 人CD3/CD28 T细胞激活剂
ImmunoCult™ XF 人T细胞扩增培养基,500 mL
EasySep™人T细胞分选试剂盒
RoboSep™ 人T细胞分选试剂盒


 EasySep™小鼠TIL(CD45)正选试剂盒
EasySep™小鼠TIL(CD45)正选试剂盒






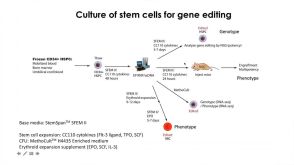
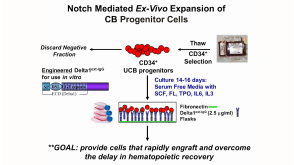



 沪公网安备31010102008431号
沪公网安备31010102008431号