若您需要咨询产品或有任何技术问题,请通过官方电话 400 885 9050 或邮箱 info.cn@stemcell.com 与我们联系
- 首页
- Scientific Resources
欢迎您关注STEMCELL Technologies的微信公众平台
扫描二维码或搜索微信号STEMCELLTech,即可关注我们的微信平台,第一时间接收丰富的技术资源和最新的活动信息。
如您有任何问题,欢迎发消息给STEMCELLTech微信公众平台,或与我们通过电话/邮件联系:400 885 9050 INFO.CN@STEMCELL.COM。
在线联系

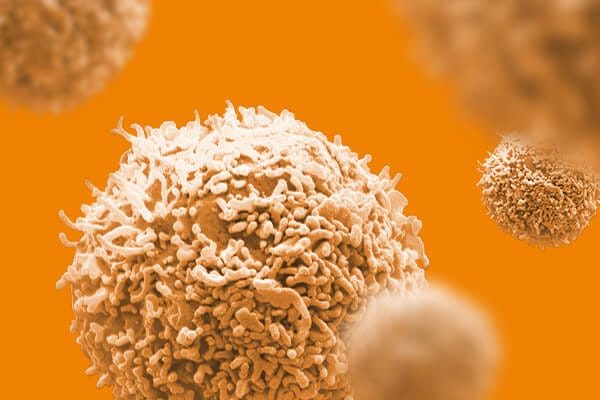
 EasySep™小鼠TIL(CD45)正选试剂盒
EasySep™小鼠TIL(CD45)正选试剂盒




 沪公网安备31010102008431号
沪公网安备31010102008431号