搜索结果: 'methocult media formulations for human hematopoietic cells serum containing'
-
 Column-Free Enrichment of EPCAM Positive and CD49F Positive Human Mammary Epithelial Progenitor Cells
Column-Free Enrichment of EPCAM Positive and CD49F Positive Human Mammary Epithelial Progenitor Cells产品类型:
Conference:
AACR 2003
产品号#:
产品名:
-
 50:26
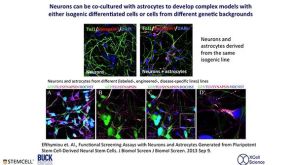
From Pluripotent Stem Cells to Neurons and Astrocytes - Modeling Human Neurological Disease
50:26
From Pluripotent Stem Cells to Neurons and Astrocytes - Modeling Human Neurological Disease产品类型:
产品号#:
05833
05835
05839
78128
78128.1
78128.2
78134
78134.1
78134.2
78136
78136.1
78136.2
78139
78139.1
78139.2
78142
78142.1
78142.2
78151.1
78151.2
78152
产品名:
STEMdiff™神经前体细胞培养基
STEMdiff™ 神经诱导培养基
STEMdiff™ 神经诱导培养基
重组人FGF-8A
重组人FGF-8A
重组人FGF-8A
重组人bFGF细胞因子, ACF
人重组bFGF,ACF
人重组bFGF,ACF
人重组EGF,ACF
人重组EGF,ACF
人重组EGF,ACF
重组人GDNF细胞因子, ACF
人重组GDNF,ACF
人重组GDNF,ACF
重组人IGF-I细胞因子, ACF
人重组IGF-I,ACF
人重组IGF-I,ACF
重组人NGF - β, ACF
重组人NGF - β, ACF
重组人PDGF-AA, ACF
发布日期: 7/31/15 -
 5:06
How to Reprogram Fibroblasts into Human Induced Pluripotent Stem (iPS) Cells Using ReproRNA™-OKSGM
5:06
How to Reprogram Fibroblasts into Human Induced Pluripotent Stem (iPS) Cells Using ReproRNA™-OKSGM产品类型:
产品号#:
05930
05931
00224UK.3
05926
产品名:
ReproRNA™-OKSGM
ReproRNA™-OKSGM
2天的hPSCs重编程和维持培养课程 - UK - 冬季
ReproTeSR™ 重编程培养基(双组分)
发布日期: 7/15/16 -
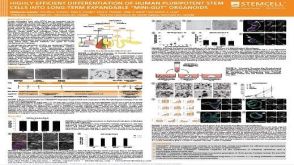 科学海报Highly Efficient Differentiation of Human Pluripotent Stem Cells into Long-term Expandable “Mini-gut” Organoids
科学海报Highly Efficient Differentiation of Human Pluripotent Stem Cells into Long-term Expandable “Mini-gut” Organoids产品类型:
Cell Culture Media and Supplements
产品号#:
06005
06010
05140
05145
产品名:
IntestiCult™ 肠道类器官生长培养基 (小鼠)
IntestiCult™ 类器官生长培养基 (人)
STEMdiff™肠道类器官试剂盒
STEMdiff™肠道类器官生长培养基
-
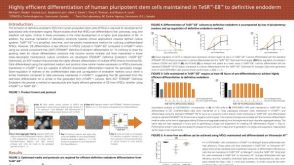 Highly Efficient Differentiation of Human Pluripotent Stem Cells Maintained in TeSR™-E8™ to Definitive Endoderm
Highly Efficient Differentiation of Human Pluripotent Stem Cells Maintained in TeSR™-E8™ to Definitive Endoderm产品类型:
Conference:
ISSCR 2014
产品号#:
05110
产品名:
STEMdiff™权威内胚层检测试剂盒
-
 科学海报Fast and Easy Isolation of CD27-Positive Human Memory B Cells Using EasySep™ Releasable RapidSpheres™
科学海报Fast and Easy Isolation of CD27-Positive Human Memory B Cells Using EasySep™ Releasable RapidSpheres™产品类型:
Cell Isolation Products
Conference:
AAI 2017
产品号#:
17864
产品名:
EasySep™ 人记忆B细胞分选试剂盒
-
 科学海报Generation of Functional Cell Types within Spinal Cord Organoids from Human Pluripotent Stem Cells
科学海报Generation of Functional Cell Types within Spinal Cord Organoids from Human Pluripotent Stem Cells产品类型:
Cell Culture Media and Supplements
产品号#:
产品名:
-
 科学海报Stroma-Free, Serum-Free Differentiation and Expansion of Hematopoietic Progenitors to the T Cell Lineage
科学海报Stroma-Free, Serum-Free Differentiation and Expansion of Hematopoietic Progenitors to the T Cell Lineage产品类型:
Cell Culture Media and Supplements
Conference:
ISEH 2016
产品号#:
09605
产品名:
StemSpan™ SFEM II
-
 Aggrewell™ 400, Aggrewell™ 800, and Aggrewell™ Medium Provide a Platform for Generation and Culture of Human Embryoid Bodies of Defined Sizes
Aggrewell™ 400, Aggrewell™ 800, and Aggrewell™ Medium Provide a Platform for Generation and Culture of Human Embryoid Bodies of Defined Sizes产品类型:
Conference:
ISSCR 2010
产品号#:
产品名:


 EasySep™小鼠TIL(CD45)正选试剂盒
EasySep™小鼠TIL(CD45)正选试剂盒






 沪公网安备31010102008431号
沪公网安备31010102008431号