产品号 #100-0620_C
无血清、无牛垂体提取物(BPE)的培养基,适用于人原代支气管上皮细胞或人气道上皮细胞分化为成熟的顶端朝外型气道类器官。
若您需要咨询产品或有任何技术问题,请通过官方电话 400 885 9050 或邮箱 info.cn@stemcell.com 与我们联系。
无血清、无牛垂体提取物(BPE)的培养基,适用于人原代支气管上皮细胞或人气道上皮细胞分化为成熟的顶端朝外型气道类器官。
无血清、无牛垂体提取物(BPE)的培养基,适用于人原代支气管上皮细胞或人气道上皮细胞分化为成熟的顶端朝外型气道类器官。
在15天内,从人支气管上皮细胞(HBECs)或人气道上皮细胞(HAECs)生成顶端朝外型气道类器官,该过程采用简便且可扩增的无Matrigel®的培养方案。这些类器官能够提供气道上皮的顶端面,并可用于在体外3D模型系统中进行传染病建模或高通量药物筛选。
使用PneumaCult™顶端朝外型气道类器官培养基,您可以在AggreWell™400 24孔板(产品号 #34415)中培养纤毛细胞的极化气道类器官,且无需使用细胞培养小室。为确保实验的可重复性,本培养基不含血清或牛垂体提取物(BPE)。
为获得更好的实验结果,建议分化前先使用PneumaCult™-NGEx 培养基(产品号 #100-1505)扩增您的HAECs。
更多关于肺类器官的信息,请访问我们的学习中心。
分类
专用培养基
细胞类型
气道细胞,上皮细胞
种属
人
应用
细胞培养,分化,类器官培养
品牌
PneumaCult
研究领域
疾病建模,药物发现和毒理检测,上皮细胞研究,感染性疾病(传染病),类器官,呼吸系统研究,干细胞生物学
制剂类别
无血清
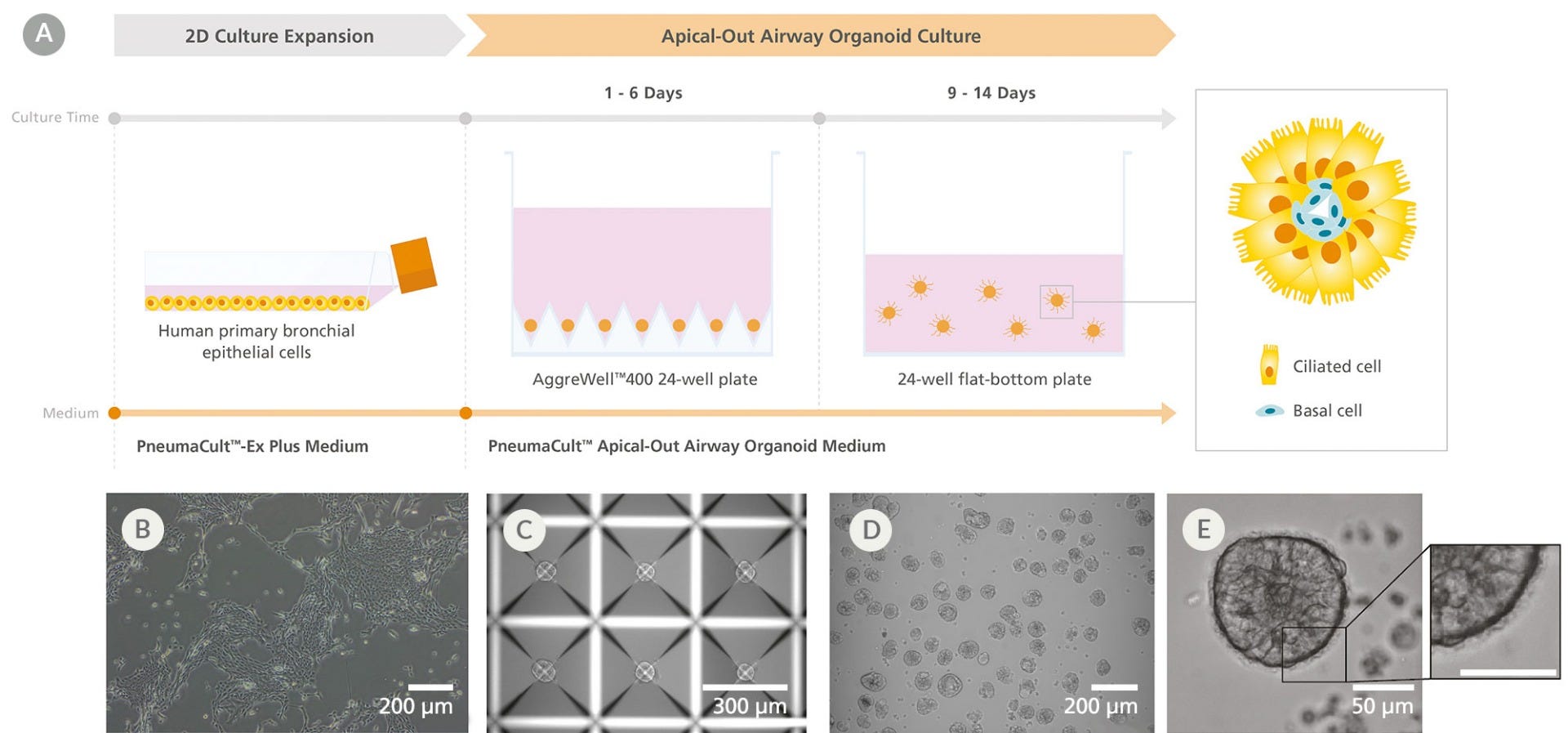
Figure 1. Generation of Apical-Out Airway Organoids Using the PneumaCult™ Apical-Out Airway Organoid Medium
(A) Workflow showing the generation of apical-out airway organoids. (B) Prior to generating apical-out airway organoids, human airway epithelial cells (HAECs) are expanded using PneumaCult™-Ex Plus medium in a two-dimensional expansion phase. (C)The HAECs are then plated into an AggreWell™400 24-well plate and allowed to aggregate for 1 - 6 days using PneumaCult™ Apical-Out Airway Organoid medium. (D) Following aggregation, the cells are dislodged from the microwells and the aggregate suspension is transferred to a 24-well flat-bottom plate and differentiated for 9 - 14 days using PneumaCult™ Apical-Out Airway Organoid medium to generate apical-out airway organoids. (E) The resulting organoids are composed of a cell-dense core containing basal cells and an epithelium that presents ciliated cells on the outer surface, indicating their successful apical-out polarity. Shown are representative brightfield images at different stages of the workflow.
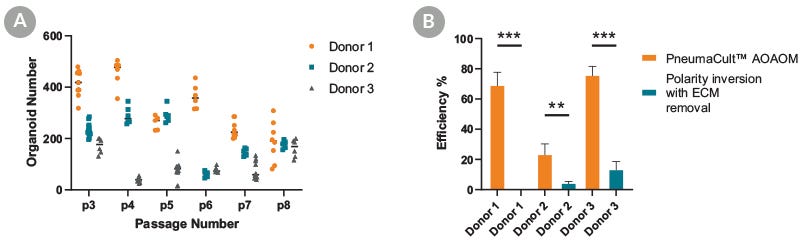
Figure 2. PneumaCult™ Apical-Out Airway Organoid Medium Supports Efficient Generation of Organoids
Apical-out airway organoids were generated by seeding 100 2D-expanded cells derived from 3 donors from passages 3 to 8. (A) Number of generated organoids per well of a 24-well plate at day 15. Data points represent measurements taken from independent wells of a 24-well plate. (B) The efficiency of two different approaches (ECM-free PneumaCult™ Apical-Out Airway Organoid medium workflow or polarity inversion following ECM removal) is expressed as the percentage of apical-out airway organoids at the end of culture relative to the total number of organoids used to seed the cultures.
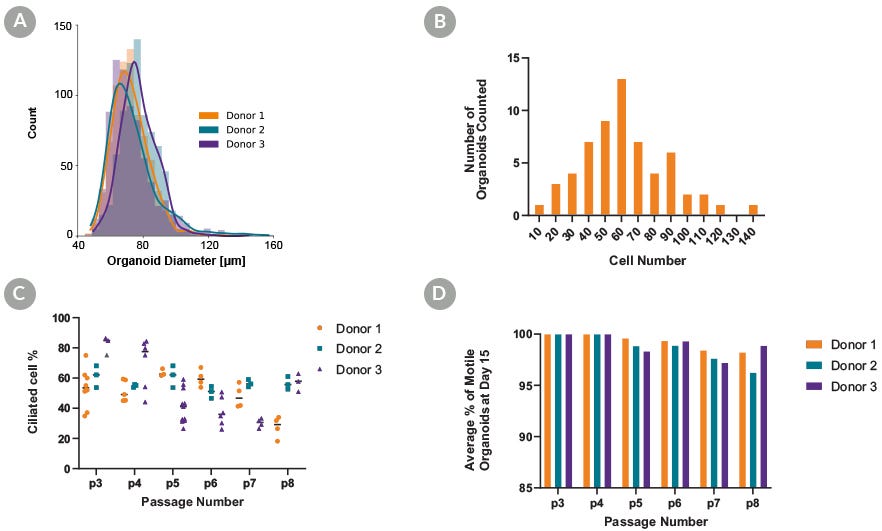
Figure 3. PneumaCult™ Apical-Out Airway Organoid Medium Supports Standardized Generation of Organoids
Absence of extracellular matrix, and aggregation using AggreWell™400 24-well plate, allows for homogeneity of the culture and the standardized generation of apical-out airway organoids that are highly similar in shape and size. For each donor at passage 3, 750 organoids were counted from 3 separate wells to generate a (A) histogram depicting the frequency of diameters in apical-out airway organoids. Organoid diameter is consistent between all donors, supporting the homogeneity of the culture. To quantify the number of cells that the organoids were composed of, passage 3 organoids were fixed, stained with DAPI, and imaged to visualize the number of nuclei and determine the (B) number of cells comprising each organoid. On average, organoids were composed of 62 cells with a 95% confidence interval of 6.77, demonstrating homogeneity at the cellular level. (C) Ciliated cell percentage of apical out organoids at day 15, (D) and the percentage of motile organoids that display beating cilia. Almost all organoids displayed functional beating cilia that allowed them to move in suspension after multiple passages, further demonstrating the homogeneity of the culture.
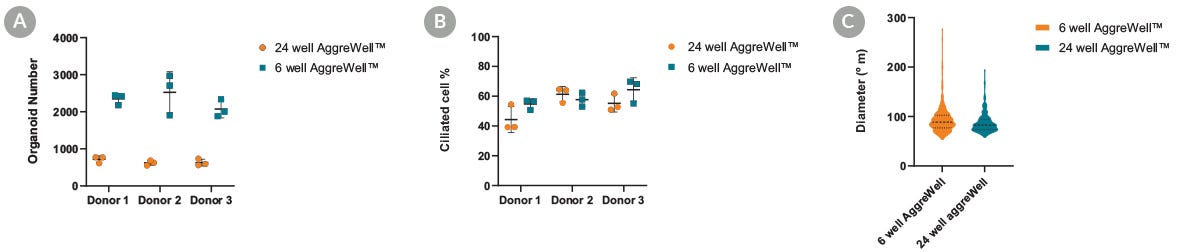
Figure 4. Apical-Out Airway Organoid Production Can Be Scaled-Up
Human airway epithelial cells (HAECs) were seeded at the same density in both the 6-well and 24-well AggreWell™ 400 plates with PneumaCult™ Apical-Out Airway Organoid medium. Data points represent individual wells analyzed on day 15 of passage number 5 for each. (A) The number of organoids obtained per well in an AggreWell™400 6-well plate was, on average, three times higher than in the 24-well plate format. (B) These organoids demonstrated similar differentiation potential and proportion of ciliated cells when compared to the 24-well format. (C) These organoids also displayed a high degree of size homogeneity between the two AggreWell™400 formats tested. This suggests that production can be scaled up without compromising the quality and functionality of the airway organoids.
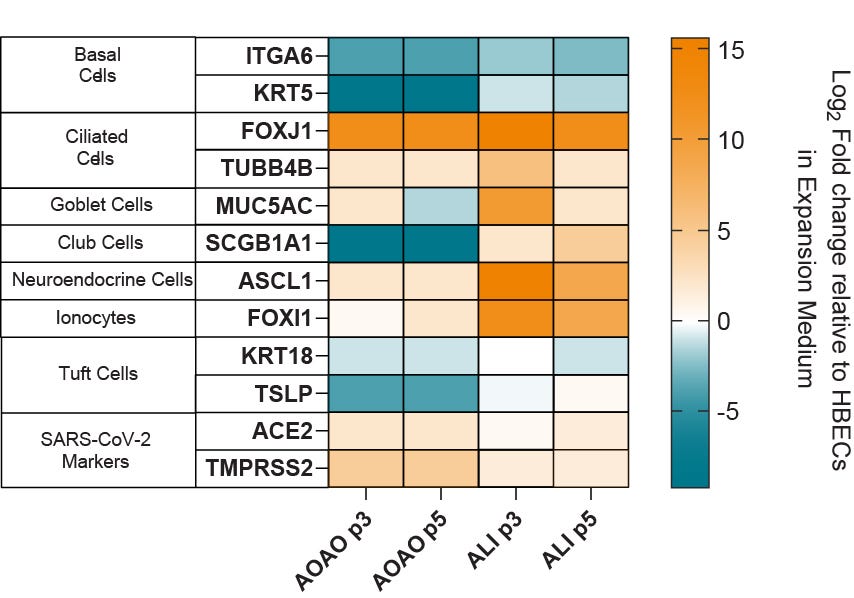
Figure 5. Gene Expression Analysis of Organoids Cultured in PneumaCult™ Apical-Out Airway Organoid Medium Shows Presence of Various Airway Cell Types
Heatmap showing fold changes in the RNA levels of common differentiation markers in apical-out airway organoids cultured in PneumaCult™ Apical-Out Airway Organoid Medium across 2 different passages, in three donors. Results were normalized against HBECs cultured in PneumaCult™-Ex Plus Medium and analyzed at passage three (n = 3). Ciliated cell markers, FOXJ1 and TUBB4B are upregulated in terminally differentiated apical-out airway organoids compared to ALI cultures. Additionally, the presence of ACE2 and TMPRSS2 markers suggests that organoids cultured in PneumaCult™ Apical-Out Airway Organoid Medium are a suitable in vitro tool to model respiratory infection by viruses such as SARS-CoV-2.
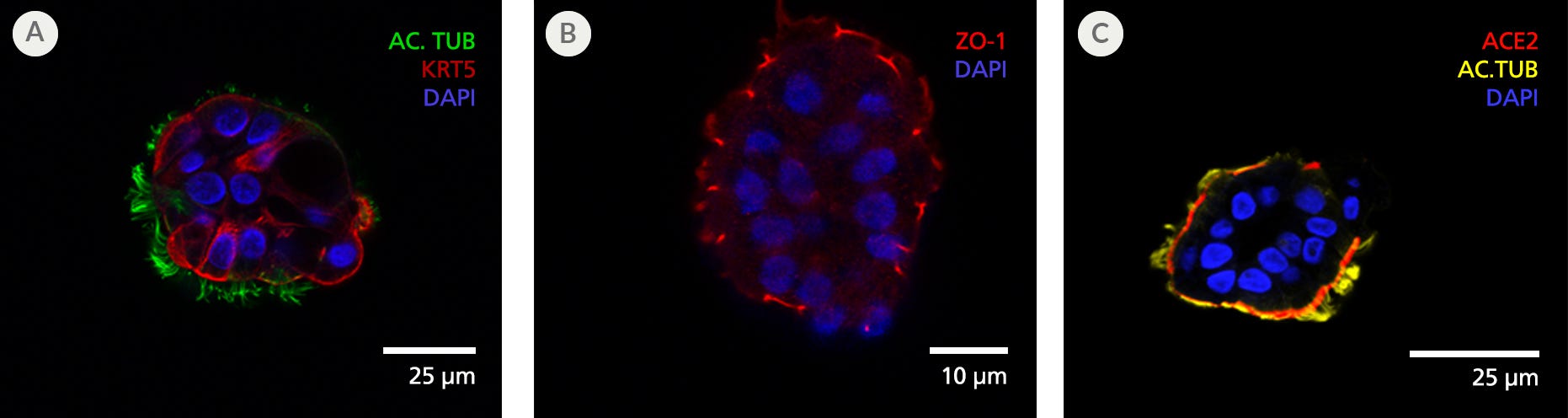
Figure 6. Terminally Differentiated Organoids Exhibit Mature Polarized Airway Epithelium
(A) Day 15 apical-out airway organoids cultured in PneumaCult™ Apical-Out Airway Organoid Medium contain ciliated cells, as confirmed by the presence of acetylated tubulin (AC. TUB; green) on the outward-facing apical cell surface, while keratin 5 (KRT5; red)-expressing basal cells were present alongside ciliated cells. (B) Cell-cell tight junction protein ZO-1 (red) can be readily visualized on the apical side of the organoid. These results indicate efficient generation of organoids across multiple passages with successful apical-out orientation. (C) Presence of ciliated cell marker acetylated tubulin (AC.TUB; yellow) and SARS-CoV-2 key entry marker ACE2 (red) is also shown, suggesting their usefulness for modeling respiratory infection from SARS-CoV-2.
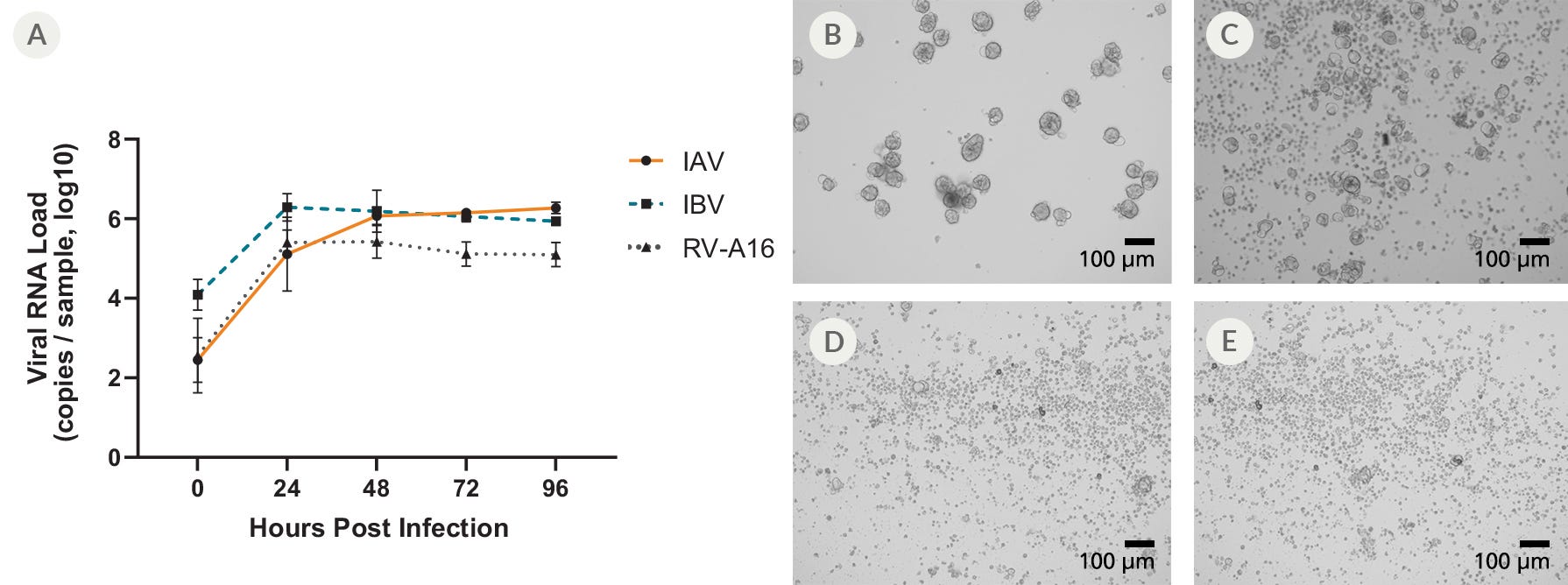
Figure 7. Apical-Out Airway Organoids Are Susceptible to Infection with Influenza A, Influenza B, and Rhinovirus A-16
(A) Terminally differentiated apical-out airway organoids were infected with Influenza A (IAV), Influenza B (IBV), and Rhinovirus-A16 (RV-A16). The viral loads in the supernatant were detected by qPCR in samples harvested at 24-hour intervals. Data points represent mean values of all biological replicates (n = 3) and error bars represent the standard deviation. When compared to (B) mock-infected organoids, the infected apical-out airway organoids showed clear signs of cytopathogenic effect (CPE) following infection with (C) RV-A16, (D) IAV, and (E) IBV 48 hours post infection. Differences in the pathogenicity of each virus could be detected; high levels of CPE were observed in apical-out airway organoids infected with IAV or IBV, whereas apical-out airway organoids infected with RV-A16 showed only moderate levels of CPE.
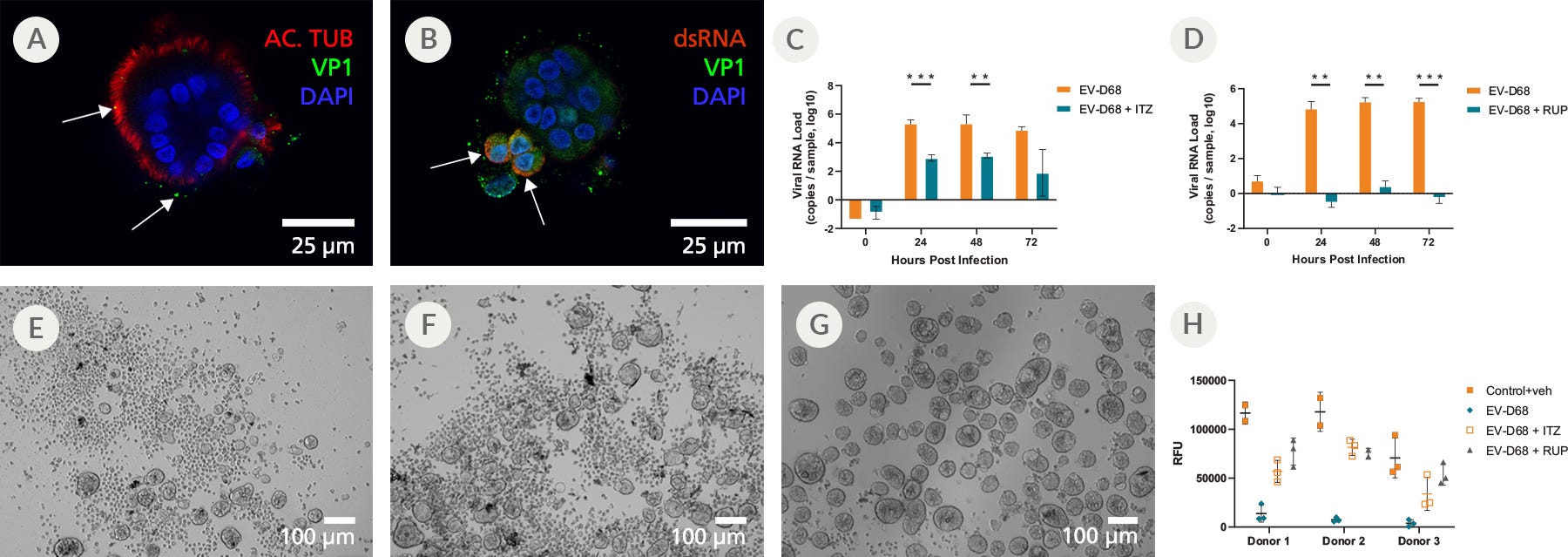
Figure 8. Mature Organoids Were Incubated with Enterovirus-D68 in the Presence or Absence of Itraconazole (ITZ) or Rupintrivir (RUP) to Study Antiviral Effects
To assess whether the PneumaCult™ Apical-Out Airway Organoid system was susceptible to infection to common respiratory viruses, day 15 differentiated organoids were infected with enterovirus-D68 (EV-D68). (A) At 0 hours post infection, the EV-D68 (VP1, green) could be seen binding to the apical surface (ciliated cells; acetylated tubulin; red). (B) After 6 hours, cells containing double-strand RNA (dsRNA; red), an intermediate stage during the viral replication cycle, can be identified. These cells are also positive for viral protein (VP1, green) and (C and D, orange bars) generate high viral RNA titers, (E) while showing evidence of cytopathogenic effect (CPE). (C, teal bars) Treatment with Itraconazole (ITZ) reduced the viral RNA levels by approximately 2 orders of magnitude and (F) slightly ameliorated the CPE levels. (D, teal bars) Treatment with Rupintrivir (RUP) completely inhibited viral replication, (G) resulting in the absence of any CPE. (H) Measuring the Relative Fluorescent Units (RFU) 72 hours post infection indicated changes in the levels of ATP and viability of the organoids, and revealed a sharp decline in viability following infection with EV-D68. A partial rescue was detected in infected apical-out airway organoids treated with ITZ, whereas those treated with RUP were almost completely rescued.
请在《产品说明书》中查找相关支持信息和使用说明,或浏览下方更多实验方案。
本产品专为以下研究领域设计,适用于工作流程中的高亮阶段。探索这些工作流程,了解更多我们为各研究领域提供的其他配套产品。
Thank you for your interest in IntestiCult™ Organoid Growth Medium (Human). Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
| 物种 | 人类 |
|---|---|
| 配方 | 无血清 |
用于在气液界面培养的人呼吸道上皮细胞的无血清和无BPE培养基
用于扩增人原代呼吸道上皮细胞的无血清和无BPE培养基
无血清和无BPE,用于人小气道上皮细胞的气液界面培养

无血清和无BPE培养基用于呼吸道类器官的高效建立和分化
人肺泡类器官扩增和分化的细胞培养基
无血清和无BPE培养基,用于人大气道、小气道和鼻上皮细胞的扩增
扫描二维码或搜索微信号STEMCELLTech,即可关注我们的微信平台,第一时间接收丰富的技术资源和最新的活动信息。
如您有任何问题,欢迎发消息给STEMCELLTech微信公众平台,或与我们通过电话/邮件联系:400 885 9050 INFO.CN@STEMCELL.COM。
在线联系