产品号 #09960_C
将人CD34+造血祖细胞扩增、分化为NK细胞
若您需要咨询产品或有任何技术问题,请通过官方电话 400 885 9050 或邮箱 info.cn@stemcell.com 与我们联系。
将人CD34+造血祖细胞扩增并分化为NK细胞
将人CD34+造血祖细胞扩增、分化为NK细胞
使用StemSpan™ NK细胞生成试剂盒,无需血清或基质细胞,即可将脐带血(CB)和骨髓(BM)中分离的CD34+细胞分化为自然杀伤(NK)细胞。该试剂盒可促进从起始的人脐带血CD34+细胞中扩增出数千个CD56+ NK细胞。
StemSpan™淋系祖细胞扩增添加物(10X)的配方含有重组人细胞因子组合和其他添加剂,当与StemSpan™ SFEM II培养基以及用StemSpan™淋系祖细胞分化包板材料(100X)包被的培养板搭配使用时,可选择性促进从人脐带血和骨髓样本中分离的CD34+细胞扩增并分化为淋系祖细胞。随后,StemSpan™ NK细胞分化添加物(100X)可将淋系祖细胞进一步分化为NK细胞。试剂盒所有组分也可单独购买,以方便您的使用。
分类
专用培养基,添加剂
细胞类型
造血干/祖细胞,NK 细胞
种属
人
应用
细胞培养,分化,扩增
品牌
StemSpan
研究领域
癌症,免疫,干细胞生物学
制剂类别
无血清
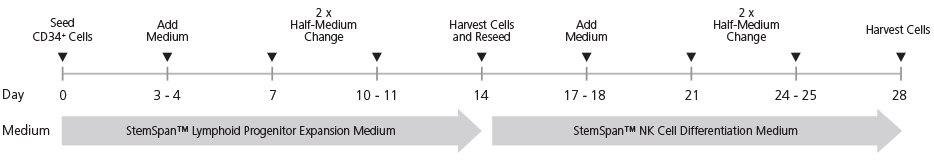
Figure 1. StemSpan™ NK Cell Generation Protocol
CB-derived CD34+ cells are seeded on day 0. Medium should be topped up after 3 - 4 days of culture followed by two half-medium changes every 3 - 4 days. On day 14, cells at the lymphoid progenitor stage are harvested and reseeded for further differentiation into NK cells. Top-up and half-medium changes should be performed every 3 - 4 days after harvest and reseed, as indicated in the figure. Note: UM729 should only be added to the NK Cell Differentiation Medium, but not the Lymphoid Progenitor Expansion Medium.
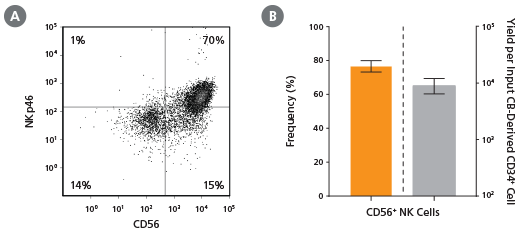
Figure 2. Frequency and Yield of CD56+ NK Cells After 28 Days of Culture
CB-derived CD34+ cells (freshly isolated or frozen) were cultured with the StemSpan™ NK Cell Generation Kit for 28 days as described. Cells were harvested and analyzed for (A,B) CD56 and (A) NKp46 expression by flow cytometry. Dead cells were excluded by light scatter profile and viability staining. (B) The average frequency of viable CD56+ NK cells on day 28 was 77%, with ~9,000 CD56+ cells produced per input CB-derived CD34+ cell. Shown are means with 95% confidence intervals (n = 45: 23 freshly isolated and 22 frozen CD34+ cell samples). BM-derived CD34+ cells were also differentiated into NK cells using the StemSpan™ NK Cell Generation Kit. The yield of NK cells from BM HSPCs is typically lower than with CB, averaging ~75 per input CD34+ cell (n = 3, data not shown).
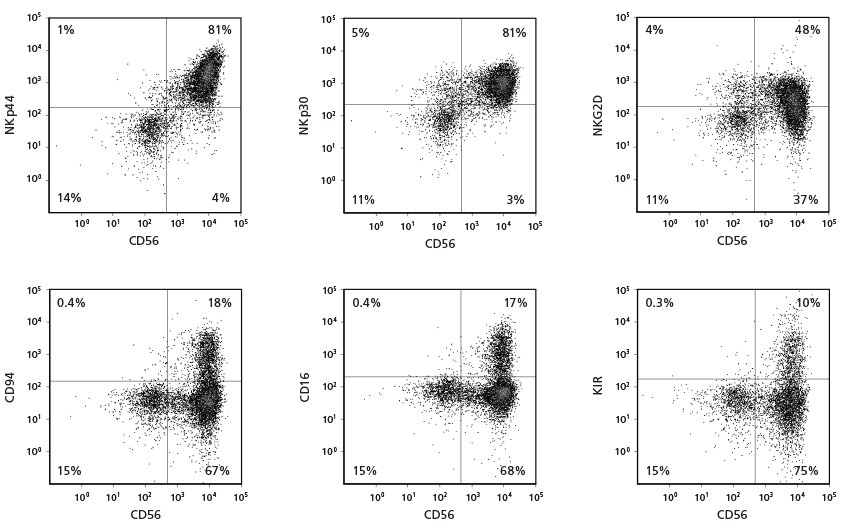
Figure 3. Cell Surface Marker Expression on CD56+ NK Cells After 28 Days of Culture
CB-derived CD34+ cells were cultured with the StemSpan™ NK Cell Generation Kit for 28 days. The differentiated cells were harvested and analysed by flow cytometry for the expression of CD56, NKp44, NKp30, NKG2D, CD94, CD16, and KIR. Staining for KIR molecules was performed using a combination of two clones for the antibody, 180704 and HP-MA4, as each recognizes a distinct subset of KIR molecules.
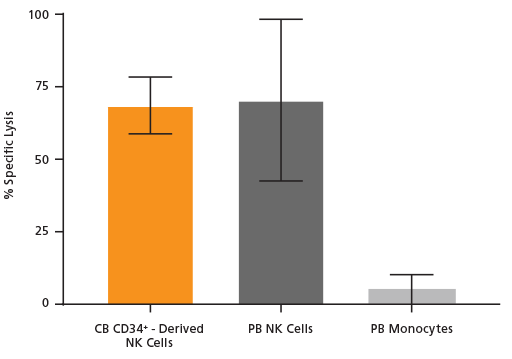
Figure 4. Cultured NK Cells Exhibit Cytotoxicity Toward K562 Cell Line
NK cells were generated from CB-derived CD34+ cells over 28 days using the protocol in Figure 1. On day 28, cells were harvested, stained for CD56, and viable CD56+ cells were counted. K562 cells were incubated with 8 μM calcein AM at 37°C for 1 hour and then washed twice. CD56+ NK cells were then combined with 10,000 of these calcein AM-labeled K562 target cells at an Effector:Target ratio of 5:1 in U-bottom 96-well plates and co-cultured at 37°C for 4 hours. Adult peripheral blood (PB) NK cells and monocytes isolated using EasySep™ were used as positive and negative controls, respectively. PB NK cells were cultured overnight with the NK Cell Differentiation Supplement and SFEM II, while PB monocytes were cultured overnight in SFEM II only. To detect spontaneous release, control wells containing only calcein AM-labeled K562 target cells were set up. The labeled K562 cells were treated with 1% Triton™ X-100 to measure maximum release. After incubation, plates were centrifuged at 500 x g for 5 minutes and 100 μL of supernatant was transferred to black plates and analyzed using a SpectraMax® microplate reader (excitation 485 nm/emission 530 nm). Results are expressed as % specific lysis: [(test release - spontaneous release) x 100] / (maximum release - spontaneous release). CB CD34+-derived NK cells show similar killing activity toward K562 target cells compared to PB NK cells. Shown are means ± SD (CB CD34+-derived NK cells: n = 18, PB NK cells and monocytes: n = 7).
请在《产品说明书》中查找相关支持信息和使用说明,或浏览下方更多实验方案。
本产品专为以下研究领域设计,适用于工作流程中的高亮阶段。探索这些工作流程,了解更多我们为各研究领域提供的其他配套产品。
Thank you for your interest in IntestiCult™ Organoid Growth Medium (Human). Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
| 物种 | 人类 |
|---|---|
| 配方 | 无血清 |
用于培养和扩增造血细胞的无血清培养基
用于人CD34+细胞扩增及分化为淋系祖细胞的添加物

用于淋系祖细胞扩增与分化的包板材料

用于淋系祖细胞向NK细胞分化的添加物

小鼠Monoclonal IgG1抗体,抗人CD56 (NCAM)

冻存的人原代细胞
扫描二维码或搜索微信号STEMCELLTech,即可关注我们的微信平台,第一时间接收丰富的技术资源和最新的活动信息。
如您有任何问题,欢迎发消息给STEMCELLTech微信公众平台,或与我们通过电话/邮件联系:400 885 9050 INFO.CN@STEMCELL.COM。
在线联系