关于我们
-
 STEMCELL Submits Drug Master File for mTeSR™1, the Most Widely Published Cell Culture Medium for Human Pluripotent Stem Cells STEMCELL Technologies announced it has filed a Type II Drug Master File (DMF) to the Food and Drug Administration (FDA) for the Cellular Therapy Ancillary Material mTeSR™1 and has been issued DMF# 18339
STEMCELL Submits Drug Master File for mTeSR™1, the Most Widely Published Cell Culture Medium for Human Pluripotent Stem Cells STEMCELL Technologies announced it has filed a Type II Drug Master File (DMF) to the Food and Drug Administration (FDA) for the Cellular Therapy Ancillary Material mTeSR™1 and has been issued DMF# 18339 -
 The Science Fair Foundation of BC Announces STEMCELL Technologies as New Title Sponsor of the Fun Run The Science Fair Foundation of BC is excited to welcome STEMCELL Technologies as the new title sponsor of The STEMCELL Science Fair Fun Run
The Science Fair Foundation of BC Announces STEMCELL Technologies as New Title Sponsor of the Fun Run The Science Fair Foundation of BC is excited to welcome STEMCELL Technologies as the new title sponsor of The STEMCELL Science Fair Fun Run -
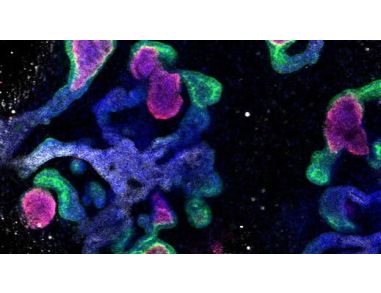 STEMCELL Technologies Signs Exclusive License to Commercialize Human Pluripotent Stem Cell-Derived Kidney Organoid Culture System STEMCELL Technologies has today announced that they have signed an exclusive license agreement with Brigham and Women's Hospital for rights to commercialize technologies for the generation of human pluripotent stem cell-derived kidney organoids
STEMCELL Technologies Signs Exclusive License to Commercialize Human Pluripotent Stem Cell-Derived Kidney Organoid Culture System STEMCELL Technologies has today announced that they have signed an exclusive license agreement with Brigham and Women's Hospital for rights to commercialize technologies for the generation of human pluripotent stem cell-derived kidney organoids


 EasySep™小鼠TIL(CD45)正选试剂盒
EasySep™小鼠TIL(CD45)正选试剂盒





