搜索结果: 'methocult media formulations for human hematopoietic cells serum containing'
-
 1:21
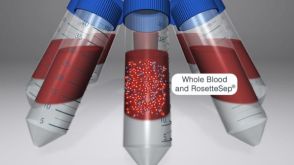
Isolate Highly Purified Cells for HLA Analysis with RosetteSep™ Immunodensity Cell Isolation
1:21
Isolate Highly Purified Cells for HLA Analysis with RosetteSep™ Immunodensity Cell Isolation产品类型:
产品号#:
15122
15162
15127
15167
15026
15066
15061HLA
15081HLA
15022
15062
15024
15064
15361
15023
15063
15126
15166
15128
15168
15129
15169
15223
15263
15725
15621
15661
15622
15662
15623
15663
15624
15664
15021
产品名:
RosetteSep™ 人CD45去除抗体混合物
RosetteSep™人CD45去除抗体混合物
含抗CD36的RosetteSep™ CTC富集抗体混合物
含抗CD36的 RosetteSep™ CTC富集抗体混合物
RosetteSep™ 人造血祖细胞富集抗体混合物
RosetteSep™人造血祖细胞富集抗体混合物
RosetteSep™ HLA T细胞富集抗体混合物
RosetteSep™ HLA T细胞富集抗体混合物
RosetteSep™人CD4+ T细胞富集抗体混合物
RosetteSep™人CD4+ T细胞富集抗体混合物
RosetteSep™ 人B细胞富集抗体混合物
RosetteSep™人B细胞富集抗体混合物
RosetteSep™人 CD4+ CD127low T细胞富集抗体混合物
RosetteSep™ 人CD8+ T细胞富集抗体混合物
RosetteSep™人CD8+ T细胞富集抗体混合物
RosetteSep™ 人脐带血减积抗体混合物
RosetteSep™人脐带血减积抗体混合物
RosetteSep™人间充质干细胞富集抗体混合物
RosetteSep™人间充质干细胞富集抗体混合物
RosetteSep™人多发性骨髓瘤细胞富集抗体混合物
RosetteSep™人多发性骨髓瘤细胞富集抗体混合物
RosetteSep™ 人总淋巴细胞富集抗体混合物
RosetteSep™人总淋巴细胞富集抗体混合物
RosetteSep™DM-M密度介质
RosetteSep™ 人CD3去除抗体混合物
RosetteSep™人CD3去除抗体混合物
RosetteSep™ 人CD4去除抗体混合物
RosetteSep™人CD4去除抗体混合物
RosetteSep™ 人CD8去除抗体混合物
RosetteSep™人CD8去除抗体混合物
RosetteSep™ 人粒细胞去除抗体混合物
RosetteSep™人粒细胞去除抗体混合物
RosetteSep™人T细胞富集抗体混合物
发布日期: 10/27/09 -
 1:05:46
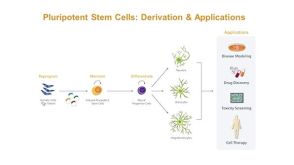
Modeling Human Neurological Disease with Induced Pluripotent Stem Cells
1:05:46
Modeling Human Neurological Disease with Induced Pluripotent Stem Cells产品类型:
产品号#:
05832
05833
05835
05839
产品名:
STEMdiff™ 神经花环选择试剂
STEMdiff™神经前体细胞培养基
STEMdiff™ 神经诱导培养基
STEMdiff™ 神经诱导培养基
发布日期: 4/25/14 -
 36:59
Derivation of Metabolically Active Hepatocytes from Human Pluripotent Stem Cells
36:59
Derivation of Metabolically Active Hepatocytes from Human Pluripotent Stem Cells产品类型:
产品号#:
05110
产品名:
STEMdiff™权威内胚层检测试剂盒
发布日期: 7/16/13


 EasySep™小鼠TIL(CD45)正选试剂盒
EasySep™小鼠TIL(CD45)正选试剂盒









 沪公网安备31010102008431号
沪公网安备31010102008431号