搜索结果: 'methocult media formulations for mouse hematopoietic cells serum containing'
-
 28:08
mTeSR™3D for Expansion and Scale Up of Human Pluripotent Stem Cell Cultures
28:08
mTeSR™3D for Expansion and Scale Up of Human Pluripotent Stem Cell Cultures产品类型:
产品号#:
27215
27250
03950
产品名:
37µm可逆滤筛,小 (15 mL)
37µm可逆滤筛,大 (50 mL)
mTeSR™3D
发布日期: 7/25/16 -
 44:40
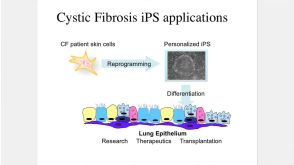
Making Lung Cells from Pluripotent Stem Cells: Disease Modeling and Future Therapies
44:40
Making Lung Cells from Pluripotent Stem Cells: Disease Modeling and Future Therapies产品类型:
产品号#:
05110
产品名:
STEMdiff™权威内胚层检测试剂盒
发布日期: 8/12/16 -
 20:24
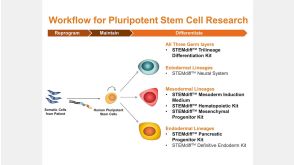
STEMdiff™ Kits for Robust and Efficient Differentiation of hPSCs to Multiple Cell Types
20:24
STEMdiff™ Kits for Robust and Efficient Differentiation of hPSCs to Multiple Cell Types产品类型:
产品号#:
05220
05221
05310
05230
05120
05240
产品名:
STEMdiff™ 中胚层诱导培养基
STEMdiff™ 中胚层诱导培养基
STEMdiff™ 造血试剂盒
STEMdiff™ 三谱系分化试剂盒
STEMdiff™胰腺祖细胞试剂盒
STEMdiff™ 间充质祖细胞试剂盒
发布日期: 6/24/16 -
 47:13
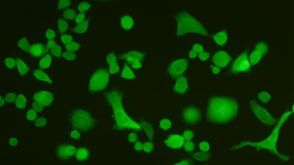
ALDEFLUOR™ and Cancer Stem Cells
47:13
ALDEFLUOR™ and Cancer Stem Cells产品类型:
产品号#:
01700
01705
01702
产品名:
ALDEFLUOR™工具
ALDEFLUOR™ DEAB试剂
ALDEFLUOR™测定缓冲液
发布日期: 11/24/09


 EasySep™小鼠TIL(CD45)正选试剂盒
EasySep™小鼠TIL(CD45)正选试剂盒












 沪公网安备31010102008431号
沪公网安备31010102008431号