搜索结果: 'methocult media formulations for mouse hematopoietic cells serum containing'
-
 44:40
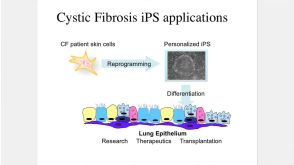
Making Lung Cells from Pluripotent Stem Cells: Disease Modeling and Future Therapies
44:40
Making Lung Cells from Pluripotent Stem Cells: Disease Modeling and Future Therapies产品类型:
产品号#:
05110
产品名:
STEMdiff™权威内胚层检测试剂盒
发布日期: 8/12/16 -
 42:24
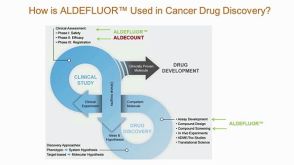
ALDH+ Cancer Precursor Cells in Drug Response Prediction
42:24
ALDH+ Cancer Precursor Cells in Drug Response Prediction产品类型:
产品号#:
01700
01705
01702
产品名:
ALDEFLUOR™工具
ALDEFLUOR™ DEAB试剂
ALDEFLUOR™测定缓冲液
发布日期: 6/25/15 -
 53:18
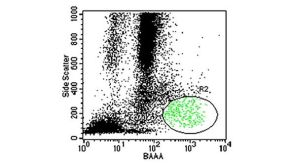
Potential Applications of ALDH Bright Cells In Regenerative Medicine
53:18
Potential Applications of ALDH Bright Cells In Regenerative Medicine产品类型:
产品号#:
01700
01705
05630
05601
05620
产品名:
ALDEFLUOR™工具
ALDEFLUOR™ DEAB试剂
EpiCult™-C 人培养基
EpiCult™-B 人培养基
MammoCult™人培养基试剂盒
发布日期: 5/27/11 -
 技术窍门Genome Editing of Human Primary T Cells Using CRISPR-Cas9
技术窍门Genome Editing of Human Primary T Cells Using CRISPR-Cas9产品类型:
Cell Engineering and Molecular Tools
细胞类型:
CD4+,CD8+,T Cells,T 细胞
产品号#:
76002
76004
76006
76010
76011
76012
76013
76016
76017
76018
76020
76021
76022
76026
200-0013
17847
100-0470
100-0471
100-0472
100-0200
100-0202
100-0204
产品名:
ArciTect™ Cas9 核酸酶
ArciTect™ Cas9 核酸酶
ArciTect™ Cas9-eGFP 核酸酶
ArciTect™ crRNA
ArciTect™ crRNA
ArciTect™ crRNA
ArciTect™ 人HPRT阳性对照试剂盒
ArciTect™ tracrRNA试剂盒
ArciTect™ tracrRNA 试剂盒
ArciTect™ tracrRNA 试剂盒
ArciTect™ 退火缓冲液 (5X)
ArciTect™ T7 核酸内切酶 I 试剂盒
ArciTect™ T7 核酸内切酶 I 试剂盒
ArciTect™ 高保真 DNA 聚合酶试剂盒
ArciTect™ sgRNA
EasySep™人TCR Alpha/Beta去除试剂盒
ArciTect™ 人 CRISPR 优化试剂盒 (APC, PE, FITC)
ArciTect™ 人 CRISPR 优化试剂盒 (APC, PE, FITC)
ArciTect™ 人 CRISPR 优化试剂盒 (APC, PE, FITC)
RoboSep™-C人CD4+ T细胞分选试剂盒
RoboSep™-C人CD8+ T细胞分选试剂盒
RoboSep™-C人T细胞分选试剂盒
-
 1:23
How to Isolate Cells with EasySep™ Column-Free Cell Separation Technology
1:23
How to Isolate Cells with EasySep™ Column-Free Cell Separation Technology产品类型:
产品号#:
17951
17951RF
17952
17952RF
17953
17953RF
19055
19055RF
17955
17955RF
18000
10985
10987
10989
10986
10988
17954
17954RF
17254
17254RF
18001
17962
17962RF
19644
19644RF
产品名:
EasySep™人T细胞分选试剂盒
RoboSep™ 人T细胞分选试剂盒
EasySep™人CD4+ T细胞分选试剂盒
RoboSep™ 人CD4+ T细胞分选试剂盒
EasySep™人CD8+ T细胞分选试剂盒
RoboSep™ 人CD8+ T细胞分选试剂盒
EasySep™人NK细胞富集试剂盒
RoboSep™ 人NK细胞富集试剂盒含滤芯吸头
EasySep™人NK细胞分选试剂盒
RoboSep™ 人NK细胞分选试剂盒
EasySep™磁极
ImmunoCult™ 树突状细胞培养试剂盒
ImmunoCult™-ACF树突状细胞培养基
ImmunoCult™树突状细胞成熟添加物
ImmunoCult™-ACF树突状细胞培养基
ImmunoCult™-ACF树突状细胞分化添加物
EasySep™人B细胞分选试剂盒
RoboSep™ 人B细胞分选试剂盒
EasySep™ 人Naïve B细胞分选试剂盒
RoboSep™ 人Naïve B细胞分选试剂盒
“The Big Easy” EasySep™磁极
EasySep™ 人静息CD4+ T细胞分选试剂盒
RoboSep™ 人静息CD4+ T细胞分选试剂盒
EasySep™大鼠B细胞分选试剂盒
RoboSep™ 大鼠B细胞分选试剂盒
发布日期: 5/3/16


 EasySep™小鼠TIL(CD45)正选试剂盒
EasySep™小鼠TIL(CD45)正选试剂盒











 沪公网安备31010102008431号
沪公网安备31010102008431号