搜索结果: 'methocult media formulations for human hematopoietic cells serum containing'
-
 1:58
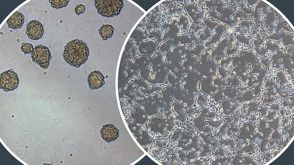
NeuroCult™ Proliferation Media
1:58
NeuroCult™ Proliferation Media产品类型:
产品号#:
05700
05702
05751
05761
产品名:
NeuroCult™ 基础培养基(小鼠&大鼠)
NeuroCult™ 扩增试剂盒 (小鼠&大鼠)
NeuroCult™ NS-A 扩增试剂盒(人)
用于小鼠和大鼠神经干细胞和祖细胞分化培养的试剂盒
发布日期: 1/19/11 -
 1:00:45
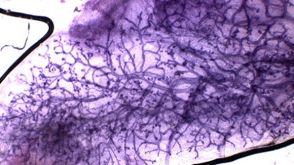
Assays for Mammary Epithelial Stem and Progenitor Cells
1:00:45
Assays for Mammary Epithelial Stem and Progenitor Cells产品类型:
产品号#:
05630
05601
05610
05620
07919
产品名:
EpiCult™-C 人培养基
EpiCult™-B 人培养基
EpiCult™-B 小鼠培养基试剂盒
MammoCult™人培养基试剂盒
Gentle Collagenase/Hyaluronidase
发布日期: 7/6/10


 EasySep™小鼠TIL(CD45)正选试剂盒
EasySep™小鼠TIL(CD45)正选试剂盒











 沪公网安备31010102008431号
沪公网安备31010102008431号