Scientific Resources
-
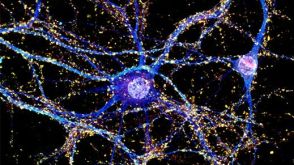 协议How to Culture Primary Rodent Neurons for MEA Analysis Using the Maestro MEA™ System
协议How to Culture Primary Rodent Neurons for MEA Analysis Using the Maestro MEA™ System产品类型:
Cell Culture Media and Supplements
研究方向:
疾病建模,神经科学,药物发现和毒理检测
-
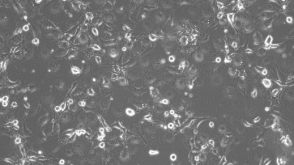 协议How to Prepare Conditioned Medium for the Expansion of Epithelial Cells
协议How to Prepare Conditioned Medium for the Expansion of Epithelial Cells产品类型:
Cell Culture Media and Supplements
研究方向:
上皮细胞研究
-
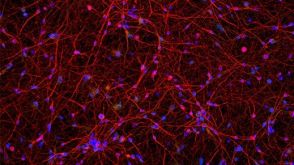 协议How to Culture Human Pluripotent Stem Cell (hPSC)-Derived Forebrain Neurons for MEA Analysis Using the Maestro MEA™ System
协议How to Culture Human Pluripotent Stem Cell (hPSC)-Derived Forebrain Neurons for MEA Analysis Using the Maestro MEA™ System产品类型:
Cell Culture Media and Supplements
研究方向:
干细胞生物学,疾病建模,神经科学,药物发现和毒理检测


 EasySep™小鼠TIL(CD45)正选试剂盒
EasySep™小鼠TIL(CD45)正选试剂盒














 沪公网安备31010102008431号
沪公网安备31010102008431号